Difference between revisions of "Management: Recommendations"
(→Criteria for a good software) |
|||
| Line 41: | Line 41: | ||
* cheap | * cheap | ||
* secure and private | * secure and private | ||
| + | * doesn't allow the modification of raw data / | ||
| + | * transfers data automatically | ||
| + | * imports metadata | ||
* time gain and not time-consuming | * time gain and not time-consuming | ||
| − | * user friendly | + | * user friendly, practical, daily use |
* operating system independent | * operating system independent | ||
* uniformity: everybody should use the same system | * uniformity: everybody should use the same system | ||
| + | * compatible and integrable with other software | ||
* flexible: customization to the CF needs | * flexible: customization to the CF needs | ||
| + | * allows the addition of extra modules (eg user database, booking, billing, ordering, budgeting) | ||
Revision as of 15:05, 19 January 2021
Think about your current management processes: what do you spend most time at? What could be automatized? A management software (commercial, open access or home-made) can help you and give you more time, which you can then invest for development, research quality improvement or customer service. Another advantage is that all information will be centralised, interconnected, traceable and searchable.
Additionally, keep records of all your samples, reagents, freezer content and so on in (Excel) databases, including all necessary information and the location of the items.
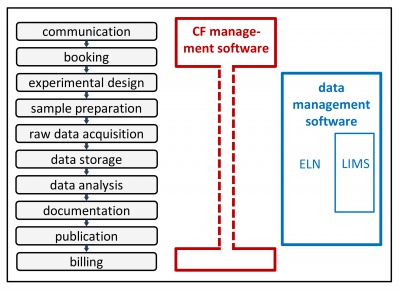
There are 2 types of management software, which are complementary: 1) CF management software and 2) data management software (see scheme).
CF management software
This type of software manages the communication with the users, the user database, the training records, the equipment booking and billing. Information and documentation regarding the experimental procedure can be added but they don’t specifically deal with this aspect. The 3 most used are:
Data management software
This type of software focuses on the documentation of the experimental procedures and data traceability. There are two different subtypes:
- electronic lab books (ELN)
- Laboratory Information Management System (LIMS) are usually linked to a machine.
Examples:
- STOCKS (open access) In addition to be an ELN, STOCKS contains also many other integrated modules (e.g. lab collections, inventory, ordering, protocols, samples, datasets). It is flexible and can be programmatically customised and expanded.
- commercial Electronic Lab Notebooks (ELN)
- Microsoft OneNote can also be adapted to be used as an ELN.
Advantages:
ELN have multiple advantages over traditional documentation. ELNs ensure documentation is complete, precise, relevant and easy to read. The data is structured, files and images can be uploaded to each entry (no glue…), links and handwritten notes can be added, and cross referencing is easy. Information is organised, easily searched, tagged and retrieved. Entries can be edited and corrected, with an automatic edit and version history of any changes made for integrity. ELNs are flexible, expandable, can be shared, backed up and take less space to store!
https://www.goldbio.com/articles/article/the-pros-and-cons-of-using-an-electronic-lab-notebook-eln
https://www.biocompare.com/Editorial-Articles/347276-Electronic-Lab-Notebooks-Are-the-Future/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4722687/
Criteria for a good software
- easy implementation, maintenance
- cheap
- secure and private
- doesn't allow the modification of raw data /
- transfers data automatically
- imports metadata
- time gain and not time-consuming
- user friendly, practical, daily use
- operating system independent
- uniformity: everybody should use the same system
- compatible and integrable with other software
- flexible: customization to the CF needs
- allows the addition of extra modules (eg user database, booking, billing, ordering, budgeting)